What is ERM?
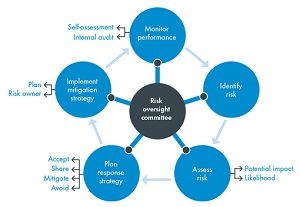 ERM or Enterprise Risk Management is defined in business as the methods and processes used by organizations to manage risks and seize opportunities related to the achievement of their objectives. ERM provides a framework for risk management, which typically involves identifying particular events or circumstances relevant to the organization’s objectives (risks and opportunities), assessing them in terms of likelihood and magnitude of impact, determining a response strategy, and monitoring progress. By identifying and pro-actively addressing risks and opportunities, business enterprises protect and create value for their stakeholders, including owners, employees, customers, regulators, and society overall.
ERM or Enterprise Risk Management is defined in business as the methods and processes used by organizations to manage risks and seize opportunities related to the achievement of their objectives. ERM provides a framework for risk management, which typically involves identifying particular events or circumstances relevant to the organization’s objectives (risks and opportunities), assessing them in terms of likelihood and magnitude of impact, determining a response strategy, and monitoring progress. By identifying and pro-actively addressing risks and opportunities, business enterprises protect and create value for their stakeholders, including owners, employees, customers, regulators, and society overall.
Why does my company need a risk management program?
The best answer I can offer is as of 2016 the average cost of a product recall was $30 million, granted major food corporations compile the amount, however I believe it best illustrates that issuing a recall can be more costly than having an ERM program in place. Product recalls can be initiated by the manufacturer or it’s client such as a retail chain, where the consumer claimed to get sick, discovered a foreign object or purchased a mis-labeled container. The retailer will typically respond very quickly by pulling the product from the shelf and then contacting the manufacturer. The retailer will expect the manufacturer to handle all of the tasks to confine, destroy or ship back products. In ERM, product issues are not just the responsibility of the manufacturing side of the business, it will involve each and every department within the organization to be most effective. Here is the link to the FDA site which posts recalls, market withdrawals & safety alerts.
What assessments should be made to mitigate a product recall?
-
- Allergens
-
- Raw materials
-
- Supplier’s product contamination
-
- Supply chain
-
- Sanitation
-
- Corporate culture
-
- Non conforming products
-
- Complaints
-
- Training
-
- Paperwork and documentation
-
- Regulatory warnings
-
- Environmental bacteria
- Finished goods bacteria
Does the FDA get involved?
Indeed they do and can find out in a number of ways:
-
- A company discovers a problem and contacts FDA
-
- FDA inspects a manufacturing facility and determines the potential for a recall
-
- FDA receives reports of health problems through various reporting systems
- The Center for Disease and Prevention (CDC) contacts FDA
Can manufacturers subscribing to a food safety program such as SQF still be at risk?
Yes, use Blue Bell as a case study, read by the Arthur Page Society.
Recall Classifications:
These guidelines categorize all recalls into one of three classes, according to the level of hazard involved:
Class I: Dangerous or defective products that predictably could cause serious health problems or death. Examples include: food found to contain botulinum toxin, food with undeclared allergens, a label mix-up on a life saving drug, or a defective artificial heart valve.
Class II: Products that might cause a temporary health problem, or pose only a slight threat of a serious nature. Example: a drug that is under-strength but that is not used to treat life-threatening situations.
Class III: Products that are unlikely to cause any adverse health reaction, but that violate FDA labeling or manufacturing laws. Examples include: a minor container defect and lack of English labeling in a retail food.
Be smart and responsible by implementing a Enterprise Risk Management program and keep the cost of a product recall at bay. Your shareholders, customers and reputation depend on it.
Contact Darryl to help you get started in a ERM program for your organization.
- Ethics In Using Madagascar Vanilla - November 21, 2023
- Money To Start Your Business - July 9, 2023
- Top 25 Common Myths About Ice Cream Co-Pack Manufacturers - April 22, 2023
